Safety Profile of Schedule III Buprenorphine and Schedule II Oral Opioids in Elderly with Chronic Low Back Pain: A Retrospective US Medicare Claims Analysis
Objective: This study aimed to evaluate and compare the safety of CIII buprenorphine and oral CII opioids among Medicare patients with chronic Low-Back Pain (cLBP).
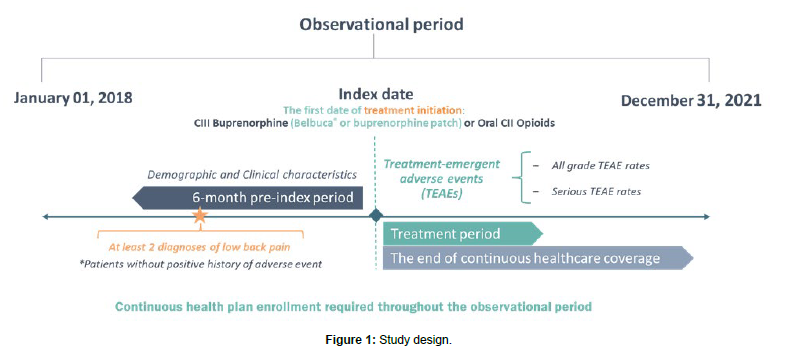
Methods: The retrospective study was conducted in Merative Medicare MarketScan® database (2018-2021). The first date of CIII buprenorphine or oral CII opioid medication prescription was defined as the index date. Patients with cLBP were observed 6 months pre-index and until the end of index treatment or the end of continuous healthcare coverage. The main outcome was the incidence of serious Treatment-Emergent Adverse Events (TEAE). Primary analysis compared CIII buprenorphine (Belbuca® and transdermal patch) with oral CII opioids, while sub-analyses compared Belbuca® to CII opioids and buprenorphine patches. Incidence Rate Ratios (IRR) and Incidence Rate Differences (IRD) (per 1,000 person-years) were reported. Propensity-Score Matching (PSM) was performed to balance differences in patients’ characteristics.
Results: CIII buprenorphine treatment (n=545 patients) was associated with significantly lower rates (p<0.050) of serious confusion (IRR=0.07), syncope (IRR=0.08), headache (IRR=0.11), urinary discomfort (IRR=0.16), constipation (IRR=0.17), cerebrovascular accident (IRR=0.18), atrial fibrillation (IRR=0.19), osteoarthritis (IRR=0.28), cellulitis (IRR=0.29), pneumonia (IRR=0.34), abdominal pain (IRR=0.45), sleep disturbances (IRD=-91.85), and hypotension (IRD=-30.62). The buprenorphine cohort (n=951 patients) more frequently experienced serious bone fractures (IRR=5.90).
Belbuca® (n=124 patients) showed significantly lower rates of serious TEAEs including fatigue (IRR=0.20), constipation (IRR=0.12), osteoarthritis (IRD=-340.08), and urinary discomfort (IRD=-194.33) than CII opioids (n=297 patients). Belbuca® had significantly lower incidence of serious dehydration (IRR=0.08), pneumonia (IRR=0.12), opioid abuse/dependence (IRD=-806.84), abdominal pain (IRD=-496.52), and appetite loss (IRD=-372.39) than patches (n=62 patients per cohort), while patches had significantly lower rates of serious osteoarthritis (IRD=867.30) and confusion (IRD=462.56).
Conclusion: Based on this retrospective claims analysis, CIII buprenorphine may have a milder safety profile than oral CII opioids for cLBP treatment. Belbuca® seems to be better tolerated than CII opioids and buprenorphine patch, based on this study.